Diagnosis of Alzheimer's disease, the study of researchers at Washington University on MTBR tau protein in cerebrospinal fluid
MTBR tau protein and Alzheimer’s diagnosis: researchers at Washington University have made a discovery that could revolutionize the diagnosis of Alzheimer’s disease.
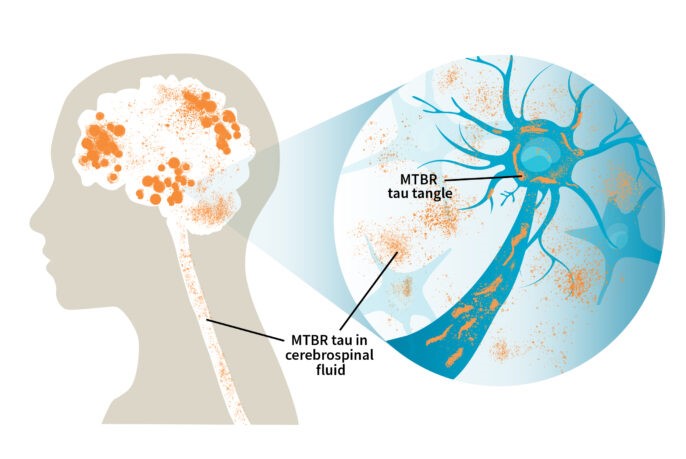
A novel form of an Alzheimer’s protein found in the fluid that surrounds the brain and spinal cord indicates what stage of the disease a person is in, and tracks with tangles of tau protein in the brain, according to a study from researchers at Washington University School of Medicine in St. Louis.
Tau tangles are thought to be toxic to neurons, and their spread through the brain foretells the death of brain tissue and cognitive decline. Tangles appear as the early, asymptomatic stage of Alzheimer’s develops into the symptomatic stage.
The discovery of so-called microtubule binding region tau (MTBR tau) in the cerebrospinal fluid could lead to a way to diagnose people in the earliest stages of Alzheimer’s disease, before they have symptoms or when their symptoms are still mild and easily misdiagnosed.
It also could accelerate efforts to find treatments for the devastating disease, by providing a relatively simple way to gauge whether an experimental treatment slows or stops the spread of toxic tangles.
The study is published Dec. 7 in the journal Brain.
“This MTBR tau fluid biomarker measures tau that makes up tangles and can confirm the stage of Alzheimer’s disease by indicating how much tau pathology is in the brains of Alzheimer’s disease patients,” said senior author Randall J. Bateman, MD, the Charles F. and Joanne Knight Distinguished Professor of Neurology.
Bateman treats patients with Alzheimer’s disease on the Washington University Medical Campus.
“If we can translate this into the clinic, we’d have a way of knowing whether a person’s symptoms are due to tau pathology in Alzheimer’s disease and where they are in the disease course, without needing to do a brain scan.
As a physician, this information is invaluable in informing patient care, and in the future, to guide treatment decisions.”
Alzheimer’s begins when a brain protein called amyloid starts forming plaques in the brain.
During this amyloid stage, which can last two decades or more, people show no signs of cognitive decline.
However, soon after tangles of tau begin to spread in the neurons, people start exhibiting confusion and memory loss, and brain scans show increasing atrophy of brain tissue.
Tau tangles can be detected by positron emission tomography (PET) brain scans, but brain scans are time-consuming, expensive and not available everywhere.
Bateman and colleagues are developing diagnostic blood tests for Alzheimer’s disease based on amyloid or a different form of tau, but neither test can pin down the amount of tau tangles across the stages of disease.
MTBR tau is an insoluble piece of the tau protein, and the primary component of tau tangles.
Bateman and first author Kanta Horie, PhD, a visiting scientist in Bateman’s lab, realized that specific MTBR tau species were enriched in the brains of people with Alzheimer’s disease, and that measuring levels of the species in the cerebrospinal fluid that bathes the brain might be a way to gauge how broadly the toxic tangles have spread through the brain.
Previous researchers using antibodies against tau had failed to detect MTBR tau in the cerebrospinal fluid.
But Horie and colleagues developed a new method based on using chemicals to purify tau out of a solution, followed by mass spectrometry.
Using this technique, Horie, Bateman and colleagues analyzed cerebrospinal fluid from 100 people in their 70s. Thirty had no cognitive impairment and no signs of Alzheimer’s; 58 had amyloid plaques with no cognitive symptoms, or with mild or moderate Alzheimer’s dementia; and 12 had cognitive impairment caused by other conditions.
The researchers found that levels of a specific form — MTBR tau 243 — in the cerebrospinal fluid were elevated in the people with Alzheimer’s and that it increased the more advanced a person’s cognitive impairment and dementia were.
The researchers verified their results by following 28 members of the original group over two to nine years. Half of the participants had some degree of Alzheimer’s at the start of the study.
Over time, levels of MTBR tau 243 significantly increased in the Alzheimer’s disease group, in step with a worsening of scores on tests of cognitive function.
The gold standard for measuring tau in the living brain is a tau-PET brain scan.
The amount of tau visible in a brain scan correlates with cognitive impairment.
To see how their technique matched up to the gold standard, the researchers compared the amount of tau visible in brain scans of 35 people — 20 with Alzheimer’s and 15 without — with levels of MTBR tau 243 in the cerebrospinal fluid.
MTBR tau 243 levels were highly correlated with the amount of tau identified in the brain scan, suggesting that their technique accurately measured how much tau — and therefore damage — had accumulated in the brain.
“Right now there is no biomarker that directly reflects brain tau pathology in cerebrospinal fluid or the blood,” Horie said.
“What we’ve found here is that a novel form of tau, MTBR tau 243, increases continuously as tau pathology progresses.
This could be a way for us to not only diagnose Alzheimer’s disease but tell where people are in the disease.
We also found some specific MTBR tau species in the space between neurons in the brain, which suggests that they may be involved in spreading tau tangles from one neuron to another.
That finding opens up new windows for novel therapeutics for Alzheimer’s disease based on targeting MTBR tau to stop the spread of tangles.”
Brain awaa373Article written by Tamara Bhandari on Washington University’s official website


