Paediatric Implantable Cardioverter Defibrillator (ICD): what differences and peculiarities?
The automatic implantable defibrillator (also called ICD from the English Implantable Cardioverter Defibrillator) is a sophisticated device that saves the lives of children with serious heart rhythm disturbances
The automatic implantable defibrillator is a very sophisticated device used to prevent sudden death
Candidate patients for implantation of this device are those who:
- Have presented with a malignant ventricular arrhythmia or cardiac arrest;
- Have, due to their characteristics and their disease, a high risk of suffering a ventricular arrhythmia or cardiac arrest.
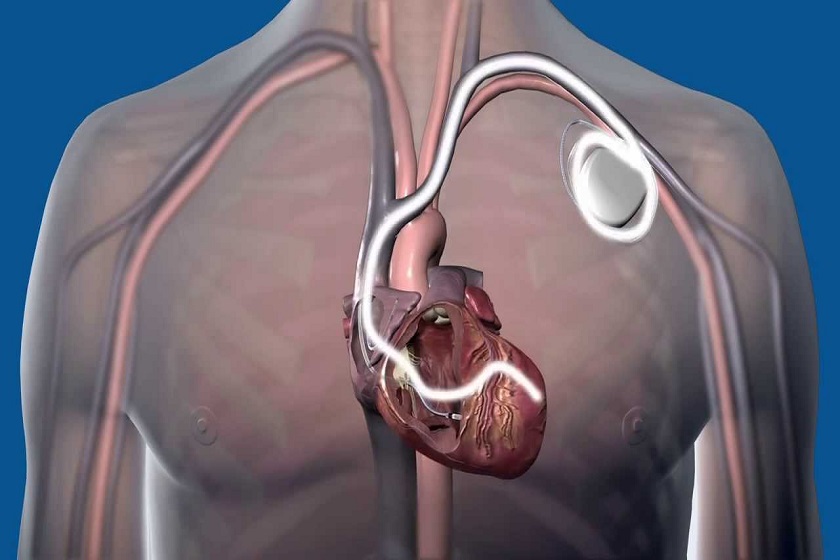
This is a small electronic device that constantly detects all heartbeats and intervenes when a serious arrhythmia occurs.
The Implantable Cardioverter Defibrillator (ICD) basically consists of 3 components:
- A battery;
- A microprocessor (a tiny computer). The battery and microprocessor are contained in a metal case somewhat larger than the size of a common matchbox;
One or more electrical wires placed in or on the heart (leads) that carry the electrical signal from the heart muscle to the defibrillator placed under the heart and vice versa.
The microprocessor is in charge of coordinating the whole, and depending on the type of device and the settings programmed by the cardiologist, the automatic implantable defibrillator is able to deliver one or more electrical therapies, the most common of which is the electric shock (also known as DC Shock), just like the normal external defibrillators found in hospitals.
Everyone has seen them, if not in hospitals, in some of the many television serials set in hospitals.
Basically, if an arrhythmia occurs and the heart rhythm accelerates abnormally (tachycardia), above a set safety limit, the risk of cardiac arrest is imminent.
The Implantable Cardioverter Defibrillator (ICD) immediately detects the arrhythmia and triggers an electric shock to normalise the heart rhythm
Implantable Cardioverter Defibrillators (ICDs), which also function as pacemakers, are able to detect abnormal slowing of the heart rhythm (bradycardia) and stimulate the heart so that it starts beating normally again, no more and no less than a pacemaker would.
The automatic implantable defibrillator generator is implanted under the skin, in the subcutis
In children weighing more than 35-40 kg, the implantation of the generator takes place in the thoracic area, under the collarbone, with the leads going to stimulate the internal surface of the heart cavities (endocardial implantation) passing through the large veins: the subclavian vein, the superior vena cava to reach the right atrium and then the right ventricle.
In children weighing less than 15-20 kg and in those in whom it is not possible to reach the cardiac chambers from the veins, the implantation is instead cardiac surgery with the placement of the leads on the external surface of the heart (epicardial implantation) and the generator placed in a subcutaneous pocket at the level of the abdomen.
Between 20 and 30-35 kg, implantation can be mixed, with leads on the outer surface of the heart (epicardial) to register malignant arrhythmia and leads reaching the inner surface of the heart via the veins to perform defibrillation.
These types of Implantable Cardioverter Defibrillator (ICD) are also able to stimulate the heart when it is unable to do so autonomously, just like a normal pacemaker.
In recent years, the traditional Implantable Cardioverter Defibrillator (ICD) has been joined by the totally subcutaneous Implantable Cardioverter Defibrillator (S-ICD), which allows defibrillation to be performed in the absence of leads placed inside the heart.
Because of its size, the S-ICD can only be implanted in paediatric patients who are slightly older, generally weighing more than 35 kg and having a Body Mass Index greater than 20.
It should be noted that subcutaneous devices (S-ICDs) are currently unable to function as pacemakers, i.e. to deliver anti-tachycardia and anti-bradycardia stimulation.
Generally, at the end of the implantation of each Implantable Cardioverter Defibrillator (ICD), it is tested whether the device is functioning correctly, inducing the arrhythmia and assessing whether the Implantable Cardioverter Defibrillator (ICD) is able to recognise and interrupt it.
CHILD HEALTH: LEARN MORE ABOUT MEDICHILD BY VISITING THE BOOTH AT EMERGENCY EXPO
The implantation of an Implantable Cardioverter Defibrillator (ICD) is a fairly safe surgery
However, like any surgery, it can have immediate complications such as: infection, allergic reactions, blood vessel damage, pulmonary collapse from haemorrhage or air infiltration, myocardial perforation and bleeding in the pacemaker pocket.
The Implantable Cardioverter Defibrillator (ICD) should be checked by physicians and technicians on a regular basis (about every 6 months), as the device may stop functioning properly over time: the cables may move or break, the heart condition may worsen, other devices interfere with the electrical signals, the battery may discharge or stop functioning properly.
Implantable Cardioverter Defibrillator (ICD) batteries can last from 5 to 7 years depending on the activity of the device
However, some functions, including the battery status, can also be controlled remotely via telemedicine.
It is also necessary to take a chest X-ray every 2 years to check the position and degree of tension of the leads, which can vary as the patient grows.
If the device intervenes (triggers an electric shock), there is no need for the family and the patient to be alarmed: in all probability the Implantable Cardioverter Defibrillator (ICD) intervened to interrupt an arrhythmia and saved the child’s life.
If there have been 1 or 2 interventions in short succession and the patient has no particular symptoms, it is advisable to contact the centre where he is being treated in order to schedule a check-up within 48 hours.
The device will provide the doctor with information about the intervention, allowing him or her to check its adequacy and correct functioning.
If, on the other hand, repeated interventions occur and if the patient experiences significant symptoms or arrhythmia and the Implantable Cardioverter Defibrillator (ICD) does not intervene, he or she should be checked at the nearest hospital as the device may be operating inappropriately or his or her heart condition may have changed.
The Implantable Cardioverter Defibrillator (ICD) wearer is provided with documentation from the Implant Centre regarding his device and how it was programmed; these documents should be carried with him at all times so that any physician is able to understand how the device works and intervene appropriately.
Read Also
Emergency Live Even More…Live: Download The New Free App Of Your Newspaper For IOS And Android
What Is The Difference Between Pacemaker And Subcutaneous Defibrillator?
What Is An Implantable Defibrillator (ICD)?
What Is A Cardioverter? Implantable Defibrillator Overview
Paediatric Pacemaker: Functions And Peculiarities
Cardiac Arrest: Why Is Airway Management Important During CPR?
RSV (Respiratory Syncytial Virus) Surge Serves As Reminder For Proper Airway Management In Children
Supplemental Oxygen: Cylinders And Ventilation Supports In The USA
Heart Disease: What Is Cardiomyopathy?
Inflammations Of The Heart: Myocarditis, Infective Endocarditis And Pericarditis
Heart Murmurs: What It Is And When To Be Concerned
Broken Heart Syndrome Is On The Rise: We Know Takotsubo Cardiomyopathy
Cardiomyopathies: What They Are And What Are The Treatments
Alcoholic And Arrhythmogenic Right Ventricular Cardiomyopathy
Difference Between Spontaneous, Electrical And Pharmacological Cardioversion
What Is Takotsubo Cardiomyopathy (Broken Heart Syndrome)?
Dilated Cardiomyopathy: What It Is, What Causes It And How It Is Treated
Heart Pacemaker: How Does It Work?


