COVID-19: rapid test, how it works and effectiveness
Rapid test: to detect SARS-CoV-2 infection, the test considered the most reliable and the reference test, according to the indications provided by the World Health Organisation (WHO), is the molecular test performed on a sample of the respiratory tract taken through a nose-pharyngeal swab
The so-called rapid antigenic test is also performed on a sample taken through a nasopharyngeal or salivary swab, but the analytical method used is different.
The two tests are therefore not comparable in terms of accuracy and reliability.
How is the rapid test performed?
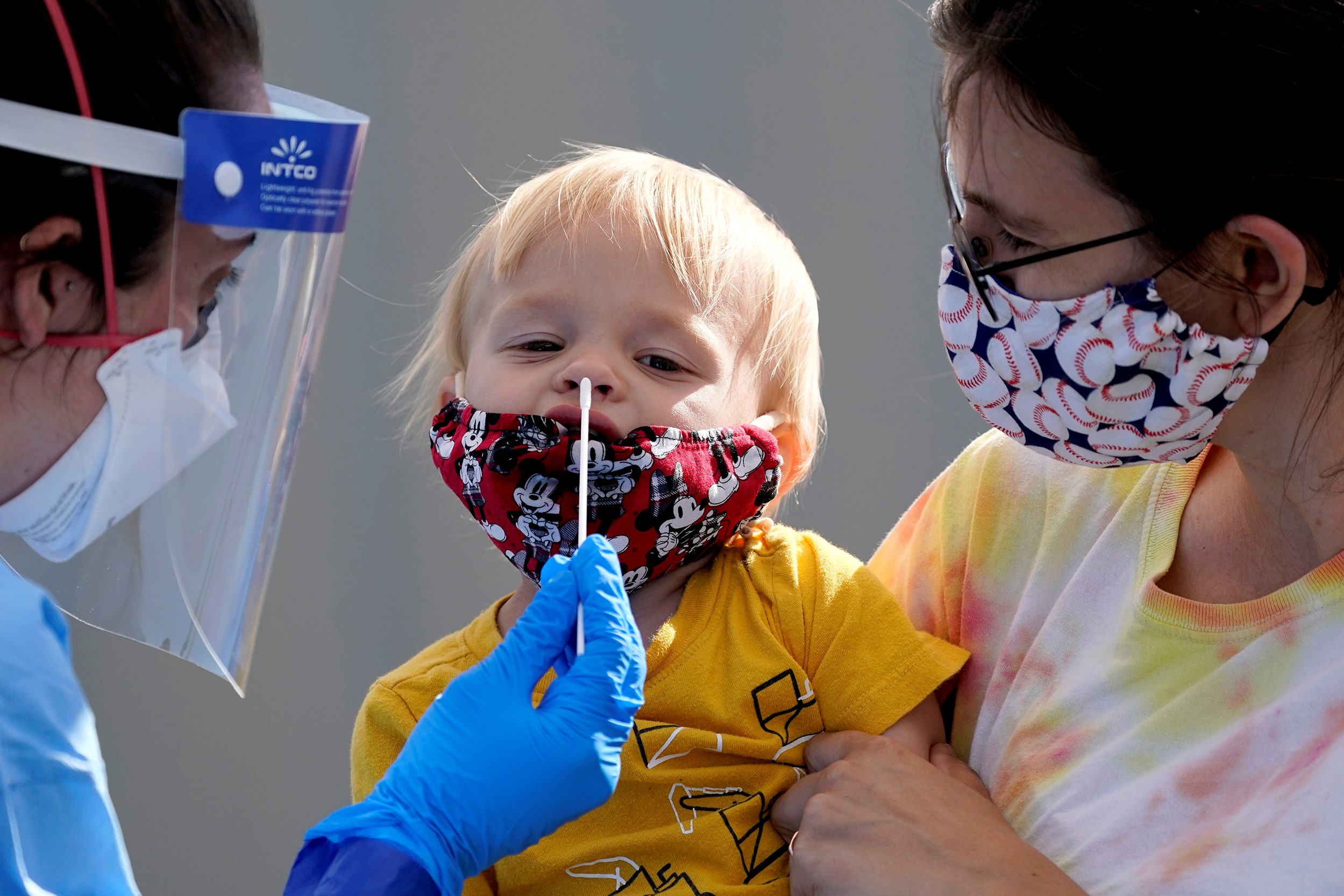
The rapid antigen test is performed on a sample taken from a nasopharyngeal swab.
Using a cotton swab inserted into each nostril of the patient, the operator removes the biological material lining the mucosa of the nasopharynx (the upper part of the respiratory tract).
As with molecular testing, the sample is taken in a few seconds, is minimally invasive and painless, although the patient may feel a sensation of discomfort when the stick comes into contact with the mucous membrane of the nose and mouth.
How does the rapid test work and when is it indicated?
The rapid antigen test looks for certain protein components of the virus (known as ‘antigens’) in swab samples and not the viral genome or parts of it, which can be detected by the molecular test.
Response times are very short, less than 90 minutes, whereas the molecular test takes about 8 hours to measure and is reported in about 24 hours.
It should be noted that the ability of the rapid antigen test to correctly detect the presence of SARS-CoV-2 viral components (sensitivity) is inferior to that of the molecular RT-PCR test, and this inferiority is particularly evident in the presence of small amounts of viral components in the test sample.
In some situations, therefore, the rapid antigen test may give false-negative results and may not allow the presence of viruses in the biological sample to be excluded with absolute certainty.
Although extremely rare, rapid antigenic testing may also give false positive results, and therefore in some categories of patients, it is necessary to confirm antigenic positivity with molecular investigations.
According to current scientific knowledge, the viral variants circulating in Italy (including Omicron) should not affect the diagnostic capacity of antigenic tests, as these detect components of the virus (the Nucleocapsid protein, N) only marginally affected by mutations.
However, it should be kept in mind that additional mutations in the N protein may emerge over time, and will need to be monitored to assess any influence on antigenic testing.
What to do if the rapid test is positive?
A positive rapid antigen test result indicates the presence of SARS-CoV-2 viral components (Nucleocapsid protein, N) in the biological sample tested, and is indicative of active viral infection.
In accordance with Decree-Law no. 229 of 30 December 2021 – “Urgent measures to contain the spread of the COVID-19 epidemic and provisions on health surveillance” – and the Circular of 30 December 2021, rapid antigenic positivity is to be considered equivalent to RT-PCR molecular test positivity.
Antigen-positive individuals should therefore be considered as having SARS-CoV-2 infection and be subject to the same reporting, isolation and quarantine rules as molecular positive individuals.
What to do if the rapid is negative?
A negative rapid antigen result suggests the absence of SARS-CoV-2 viral components (Nucleocapsid protein, N) in the biological sample tested, and is indicative of the absence of active viral infection.
In accordance with Decree-Law no. 229 of 30 December 2021 – “Urgent measures for the containment of the spread of the COVID-19 epidemic and provisions on health surveillance” – and the Circular of 30 December 2021, in non-hospital contexts, negativity to the rapid antigenic swab is to be considered equivalent to negativity to the molecular test in RT-PCR.
In hospital settings, the possible occurrence of “false negative” results requires, for certain types of patients and clinical conditions, confirmation of negative rapid antigenic results by molecular RT-PCR testing.
Read Also:
Emergency Live Even More…Live: Download The New Free App Of Your Newspaper For IOS And Android
Swabs Chaos, What To Do And When To Do It: In Italy Infectious Diseases Experts Provide Clarity
US Paediatricians Warn: 78% More Covid Cases Among Children In One Week


